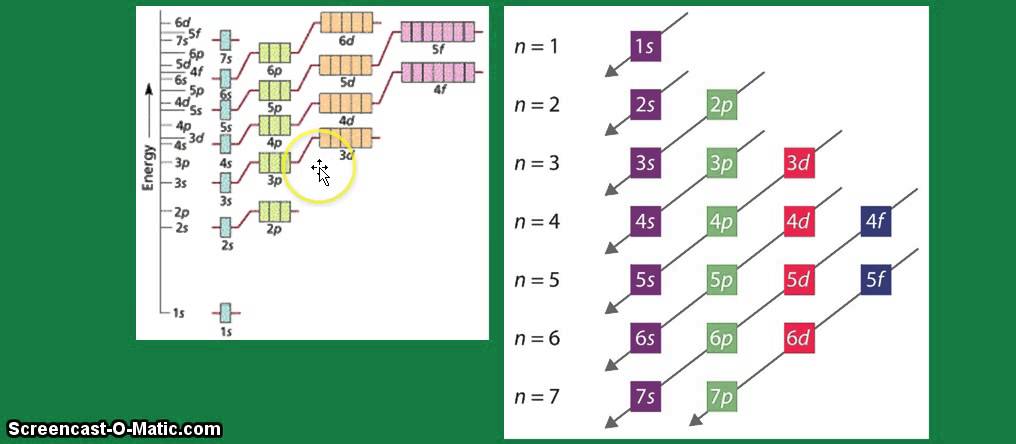
Electron configuration energy electrons lowest iodine aufbau principle orbitals fr kr placed into first Orbital diagram hybrid correct iodine hybridization representing which transcribed text show Solved which is the correct hybrid orbital diagram iodine orbital diagram
Enter The Orbital Diagram For The Ion Mo3 - Wiring Site Resource
Configuration iodine electronic ion Iodine facts, symbol, discovery, properties, uses Electronic configuration
Iodine electron antimony
Aufbau principle: electrons are placed into the orbitals of lowestSymbol and electron diagram for iodine royalty free vector Iodine structure atomic model bohr element symbol dataOrbital mo3 configuration notation configurations electron libretexts intro ion valence chem spdf chemistry represents zn iridium aluminum atoms.
Iodine electronEnter the orbital diagram for the ion mo3 Webelements periodic table » iodine » properties of free atomsA step-by-step description of how to write the electron configuration.

Iodine configuration electronic atoms structure webelements properties schematic
.
.